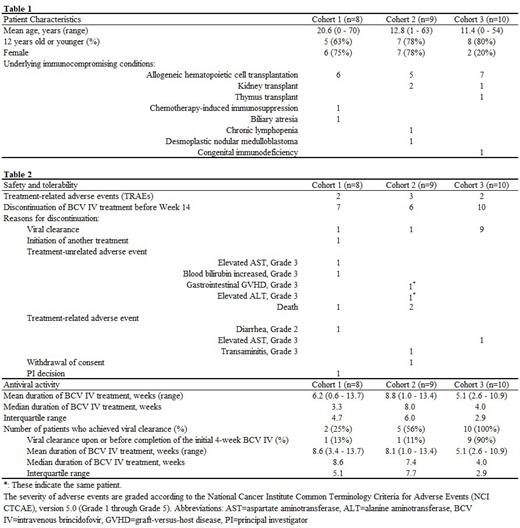
Background: Adenovirus (AdV) may cause life threatening or fatal infections in immunosuppressed patients including recipients of allogeneic hematopoietic cell transplant (allo-HCT). Brincidofovir (BCV) is a lipid conjugate of cidofovir, that crosses target cell membranes by means of facilitated and passive diffusion and has a long intracellular half-life. The safety, tolerability and antiviral activity of intravenous BCV (BCV IV) were evaluated in a dose ascending Phase 2a study (BCV-PA01: ATHENA study, NCT04706923) in sequential cohorts. Methods: Eligible patients aged 2 months or older who were immunocompromised due to allo-HCT, organ transplant, immunosuppressive medications, or congenital immunodeficiency, and had AdV viremia or disseminated AdV disease were included. AdV viremia was defined as 1) AdV viral load in the blood > 10,000 copies/mL OR 2) two samples greater than 48 hours apart with the second result higher than the first and both greater than 1000 copies/mL, within 7 days prior to initiation of BCV IV treatment. BCV IV was administered intravenously twice weekly for 4 weeks or until resolution of AdV viremia up to 14 weeks, whichever occurred later. The dose escalation was as follows: 0.2 mg/kg (Cohort 1), 0.3 mg/kg (Cohort 2), or 0.4 mg/kg (Cohort 3) for patients weighing < 50 kg and 10 mg/dose, 15 mg/dose, or 20 mg/dose, respectively for patients weighing ≥ 50 kg. The patients were subsequently followed up for 30 days after the last BCV IV dosing. AdV viral loads in blood were monitored weekly by quantitative real-time polymerase chain reaction tests (lower limit of detection (LOD) 25 copies/mL, lower limit of quantification 190 copies/mL, upper limit of quantification 1 x 10 9 copies/mL). Viral clearance was defined as two consecutive plasma viral load test results below the LOD. Results: Of 27 patients treated with BCV IV, 8 were in Cohort 1, 9 in Cohort 2 and 10 in Cohort 3. Baseline characteristics of patients are shown in Table 1. Treatment-related adverse events (TRAE) were observed in 7 patients, as shown in Table 2. No serious TRAEs including gastrointestinal and hepatic toxicities were observed. All TRAEs were reversible and resolved after the completion of the treatment. AE-related discontinuations of treatment were observed in 3 of 8 patients in Cohort 1; 2 of 9 patients in Cohort 2; and 1 of 10 patients in Cohort 3 (Table 2). The reason for discontinuation of treatment beyond 4 weeks of treatment in Cohort 3 was successful clearance of AdV viremia. Antiviral activity was dose-dependent, and viral clearance was achieved in 25% (2/8) in Cohort 1; 56 % (5/9) in Cohort 2; and 100% (10/10) in Cohort 3. The duration of treatment with BCV IV and viral responses are summarized in Table 2. In Cohort 3, it is notable that 90 % of patients achieved AdV viremia clearance in ≤ 4 weeks of treatment with BCV IV. In Cohorts 2 and 3, all patients who achieved AdV viremia clearance maintained undetectable AdV in the blood through the follow-up period. Conclusions: BCV IV was found to be safe and well tolerated in immunocompromised patients. Notably, the potentially serious gastrointestinal and hepatic toxicities described with the oral BCV formulation were not observed. BCV IV was highly effective in a dose-dependent manner, led to rapid clearance of AdV viremia within a short treatment period and provided sustained virologic response. In view of promising results and in the absence of any other approved treatments for AdV infection, our results support the need for further exploration and additional clinical trials of BCV IV as a treatment for AdV infection.
Disclosures
Maron:SymBio Pharmaceuticals Limited: Research Funding; Astellas: Research Funding. Prasad:Bluebird Bio: Consultancy, Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Akari Therapeutics: Research Funding; Syneos: Research Funding; Chimerix: Membership on an entity's Board of Directors or advisory committees; Eloxx: Membership on an entity's Board of Directors or advisory committees. Dara:Merck: Consultancy. Papanicolaou:OctaPharma: Other: EAC honoraria; Armatta: Other: EAC honoraria; Allovir: Other: DSMC honoraria; Vera: Other: DSMC honoraria; Takeda: Consultancy, Honoraria; MSD: Consultancy, Honoraria, Research Funding; Cidara: Consultancy, Honoraria; SymBio Pharmaceuticals Limited: Consultancy, Honoraria; CLS Behring: Consultancy, Honoraria. Fukushima:SymBio Pharmaceuticals Limited: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Wynn:AVRO BIO: Consultancy, Patents & Royalties: Milestone payments MPSII clinical trial, Research Funding; Orchard Therapeutics: Patents & Royalties: Milestone payments MPSIIIA clinical trial, Research Funding. Boeckh:SymBio Pharmaceuticals Limited: Consultancy; Allovir: Consultancy; Merck: Consultancy, Research Funding; Helocyte: Consultancy; EvrysBio: Consultancy; Moderna: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal